What Term Best Describes Naoh in This Reaction
Heats of Reaction Note. 1 0 M H C l until the reaction was complete.

Sustainability Free Full Text Impact Of Naoh Concentration On Deweaving Of Cotton Fabric In Aqueous Solutions Html
HNO3 aq Ba OH2 aq --.
. Saponification is a reaction in which ethanol and sodium ethanoate are produced upon treatment of ester with an alkali. CuCl 2 aq 2NaOHaq CuOH 2 s 2NaClaq A. What volume of an 800 M solution of NaOH would be needed to precipitate all copper ions from 250.
In the following reaction. What type of chemical reaction is illustrated in the following example. All that was left in the container was the salt.
Thus we can conclude that when an acid such as hcl reacts with a base such as naoh the products are a salt nacl and water h2o then neutralization is the term which best describes. NaOH HCl NaCl H2O. Basically in this type of reaction acid is neutralized by the addition of a base.
Aluminium is an amphoteric element that is it reacts with both acids and bases to produce a salt and hydrogen. The student produced a salt and water. Which best describes the chemical and phase changes that occur in a burning candle.
Four stereoisomers are produced b. The reaction in which hydrochloric acid HCl reacts with sodium hydroxide NaOH to produce water H2O and sodium chloride NaCl is a special type of double displacement reaction called a neutralization reaction. ОН NaOH Br a.
Which type of change is best for monitoring the reaction rate of the reaction below. 2Al 2NaOH 6H2O 2Na Al OH4 3H2. What are the predicted products from the following neutralization reaction.
D CH 3 COOC 2 H 5 NaOH CH 3 COONa C 2 H 5 OH. A pair of diastereomers are produced c. Chemistry questions and answers.
Saponification is the process of preparation of soap. To determine when the reaction is complete students will use a small amount of the indicator. Consider the reaction shown below.
The reaction is highly exothermic. NaOH sH2O l NaO aq H30 aq C. After the liquid left in the container was dried which of the following statements must be true.
Octane C8H18 is a major component in gasoline. For the following acid-base reaction predict which side of the equilibrium is favored. The reaction of HCl with NaOH is represented by the equation HClaq NaOHaq NaClaq H2Ol What volume of 0135 M HCl is required to titrate 398 mL of 0718 M NaOH.
Ba NO32 and H2O. The reaction does not require heat energy and the catalyst involved in the reaction is base. NaOH aq HCl aq Na OH- H Cl-.
Here sodium chloride NaCl is the salt formed due to chemical reaction between HCl and NaOH. Which of the following reactions best describes the dissolution of solid NaOH s in water. Sodium hydroxide NaOH.
Therefore this reaction is a neutralisation reaction as the acid and base. Which statement best describes the physical properties of the following amino acid. 202 CO2 2H20 a oxidized b oxidizing agent c hydrolyzed d reduced 8.
In this reaction an acid HCl and a base NaOH are reacting with each other to form a salt NaCl which is neutral in nature and water H20. What volume of 0500 M NaOH is required to neutralize 250 mL of 0250 molL HBr. HCl NaOH NaCl H 2 O The apparatus shown was used where HCl was reacted with NaOH.
Therefore its a neutralization reaction. A Na Cl ---Naci b H2O - HO Hº c Ni2 Fe --- Fe Ni d NaOH HCI --NaCl H20 7. Which of the following reactions is NOT a redox reaction.
Which half-reaction takes place at. Dissociates in H and Cl-. B c acid-base reaction electrophilic substitution elimination of HBr nucleoph ilic su bstitutio n The compound 12-dichloroethene C2H2C12 has been used as an industrial solvent for a number of compounds including fats camphor and caffeine.
MgOH2s 2 HClaq. Determine if NaOH is a suitable reagent to deprotonate the following compound. NaOHaq HC1aq NaDHaq Which term describes the action of NaOHaq on a bromoalkane.
NaOH s NaOf aqH aq b. H2SO4 aq NaOH aq Na2SO4 aq H2O l neutralization reaction. The reaction of Aluminium with Sodium Hydroxide is a Displacement reaction where one element is more reactive than the other and displaces it in a reaction.
A pair of enantiomers are produced d. This reaction involve an acid HCl reacting with a base NaOH producing a salt NaCl and water. One meso compound is produced e.
Click hereto get an answer to your question Which of the following best describes the salt formed from the neutralization reaction between aqueous solutions of NaOH and HC2H3O2 I. Which one of the following equations best describes this reaction. Naoh And Water - 17 images - solved a solution naoh aq contains 8 9 g naoh s per 100 deionizer deionized water systems suppliers ro filters ppt strong bases lioh naoh koh rboh csoh ca oh 2 pdf investigation of naoh properties production and.
One chiral compound is produced. Hydrochloric acid HCl. Na aq OH- aq H aq Cl- aq Na aq Cl- aq H20 l.
A 500 mL U B 125 mL. ML of a 400 M solution of CuCl 2. NaOH s Nat aq OH aq I d.
In an experiment to find the heat of reaction for. This is an acid-base reaction in which the acetylsalicylic acid reacts with the base sodium hydroxide to produce the salt sodium acetylsalicylate and water acid base salt water. Dissociates in Na and OH-.
CH 4 2O 2g CO 2g. In a double displacement reaction the anions of the reactants switch the cations they are associated with. The terms heat and enthalpy are interchangeable in some of these questions.
Which of the following best describes the product s of this SN2 reaction. 2 0 M N a O H with 0. In a neutralization reaction performed in lab a student mixed 0.
Which of the following terms best describes CH. The reaction of HCl with NaOH is represented by the equation HClaq NaOHaq - NaClaq H2Ol asked Jun 27 2017 in Chemistry by Christeen What volume of 0135 M HCl is required to titrate 398 mL of 0718 M NaOH.
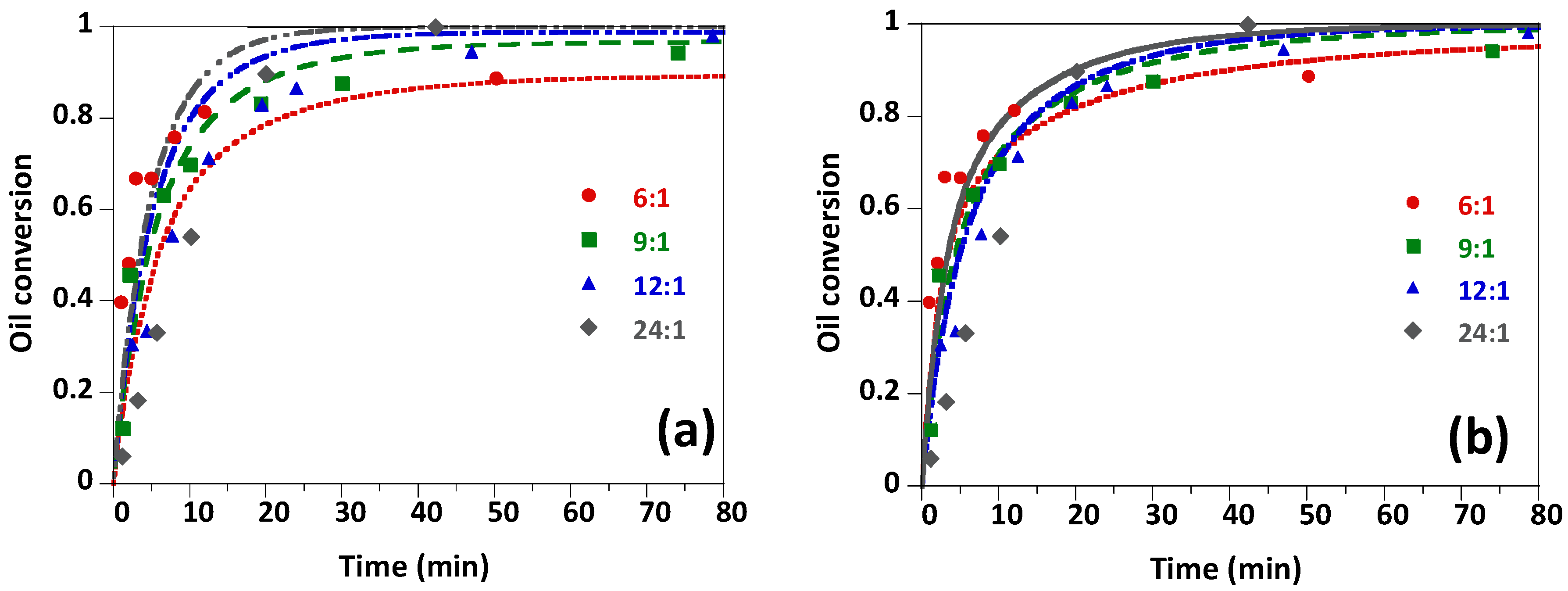
Energies Free Full Text Pseudo Homogeneous And Heterogeneous Kinetic Models Of The Naoh Catalyzed Methanolysis Reaction For Biodiesel Production Html

Sodium Hydroxide Sulfuric Acid Acid Base Neutralization Reaction Youtube
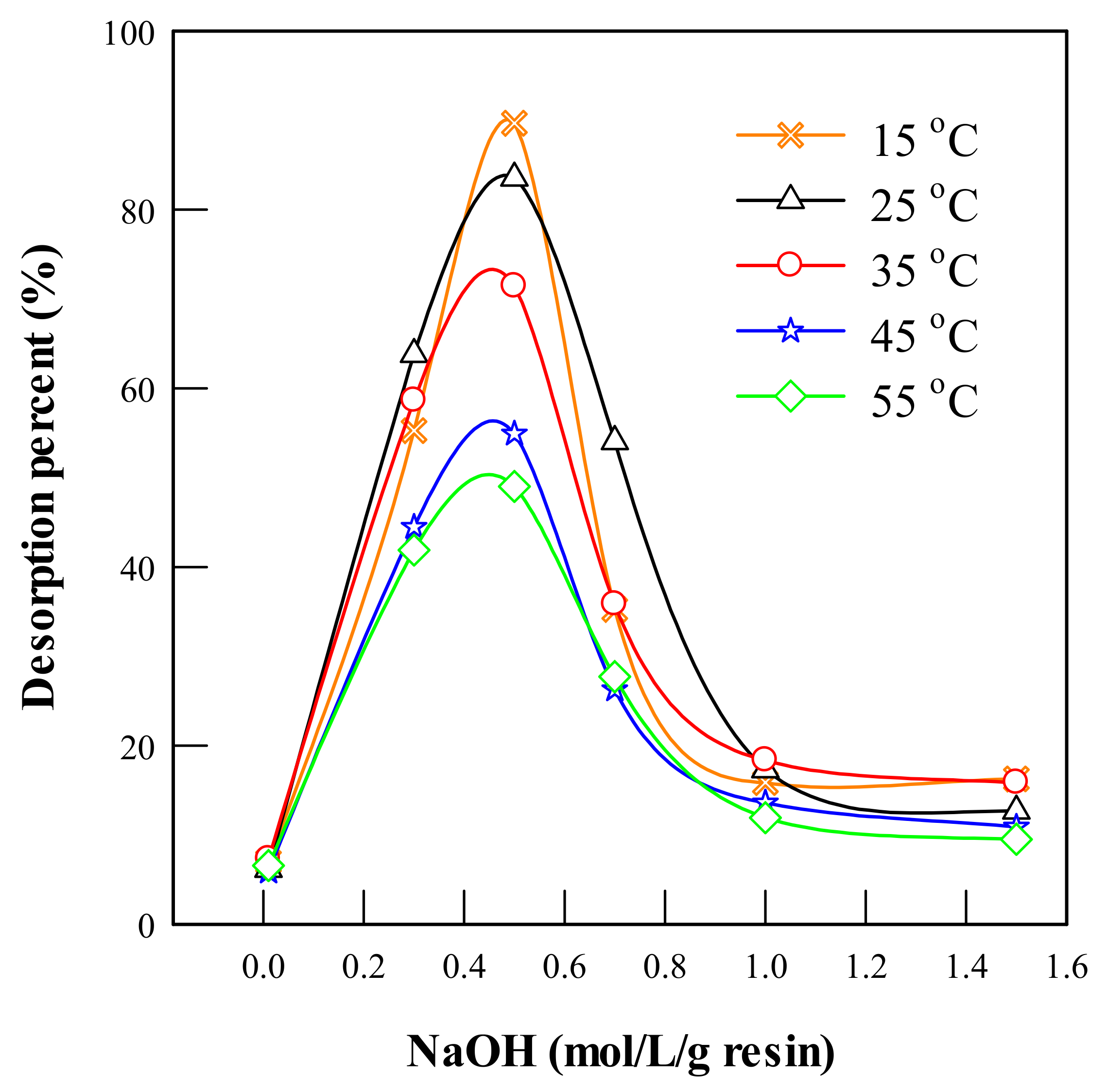
Processes Free Full Text Adsorption And Desorption Behavior Of Ectoine Using Dowex Hcr S Ion Exchange Resin Html
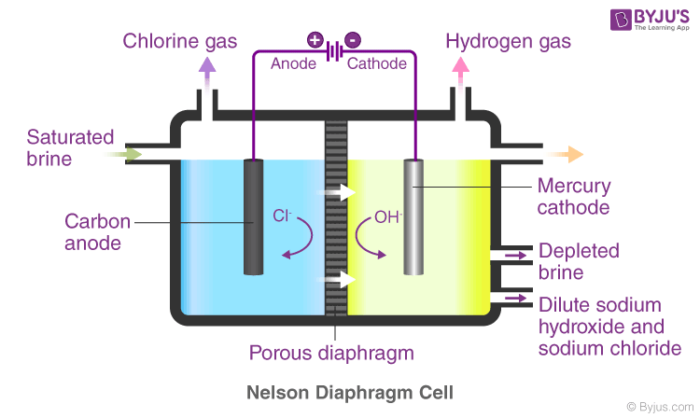
Caustic Soda Naoh Chemical Formula Properties And Uses

10 Ml Of A Solution Of Naoh Is Found To Be Completely Neutralised By 8 Ml Of A Given Solution Of Hcl If We Take 20 Ml Of The Same Solution Of

Profiles Of Ph And Orp When Naoh Was Added Into Pure Water Download Scientific Diagram

Profiles Of Ph And Orp When Naoh Was Added Into Pure Water Download Scientific Diagram

Top 10 Best Slide Phones Reviews Phone Cool Slides Phone Design

Reagents And Conditions I Naoh Ethanol Rt Stir Overnight Download Scientific Diagram

Resources Free Full Text Bacterial Nanocellulose Derived From Banana Leaf Extract Yield And Variation Factors Html

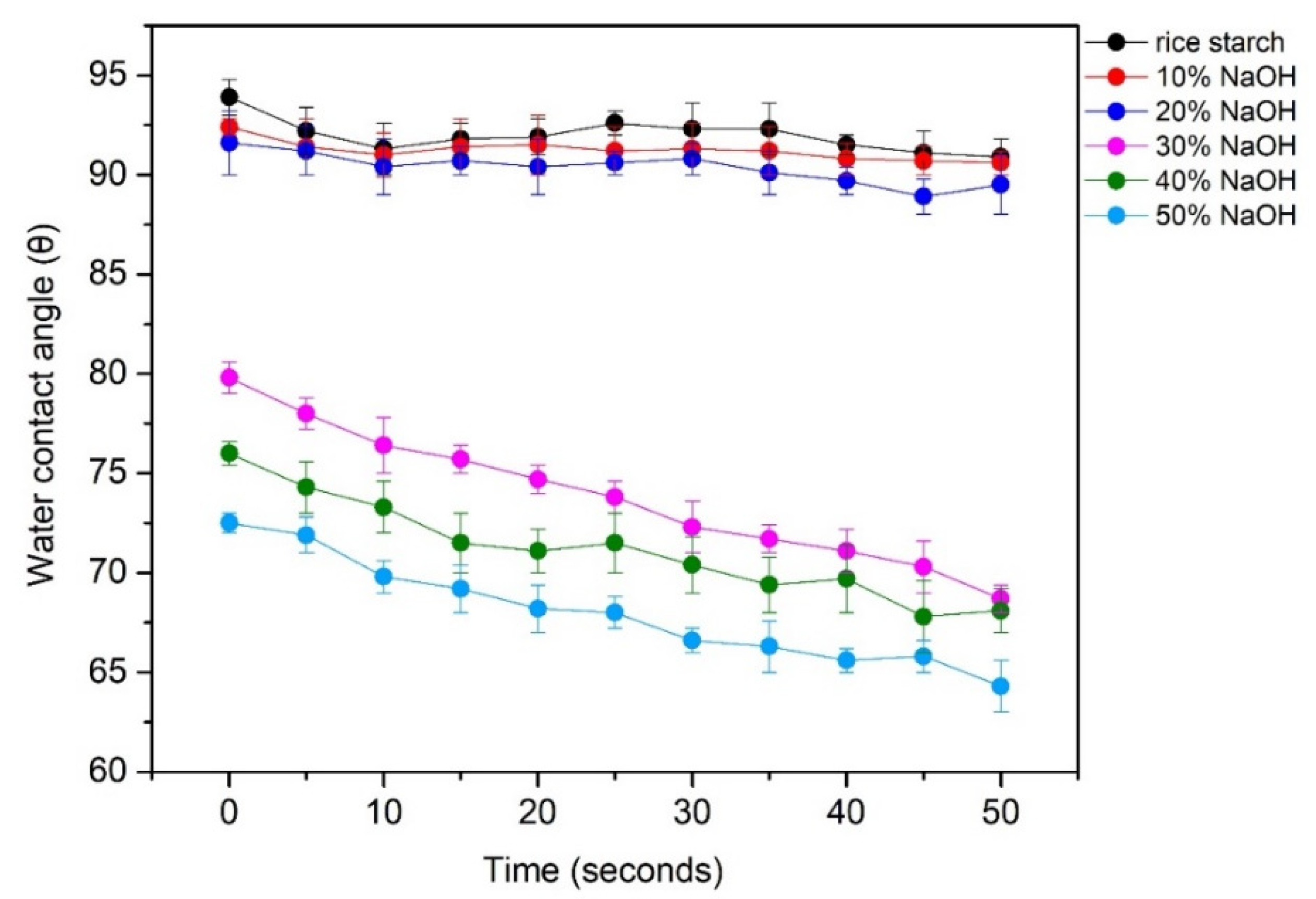
Molecules Free Full Text Morphology Mechanical And Water Barrier Properties Of Carboxymethyl Rice Starch Films Sodium Hydroxide Effect Html

Molecules Free Full Text Morphology Mechanical And Water Barrier Properties Of Carboxymethyl Rice Starch Films Sodium Hydroxide Effect Html

Molecules Free Full Text Morphology Mechanical And Water Barrier Properties Of Carboxymethyl Rice Starch Films Sodium Hydroxide Effect Html
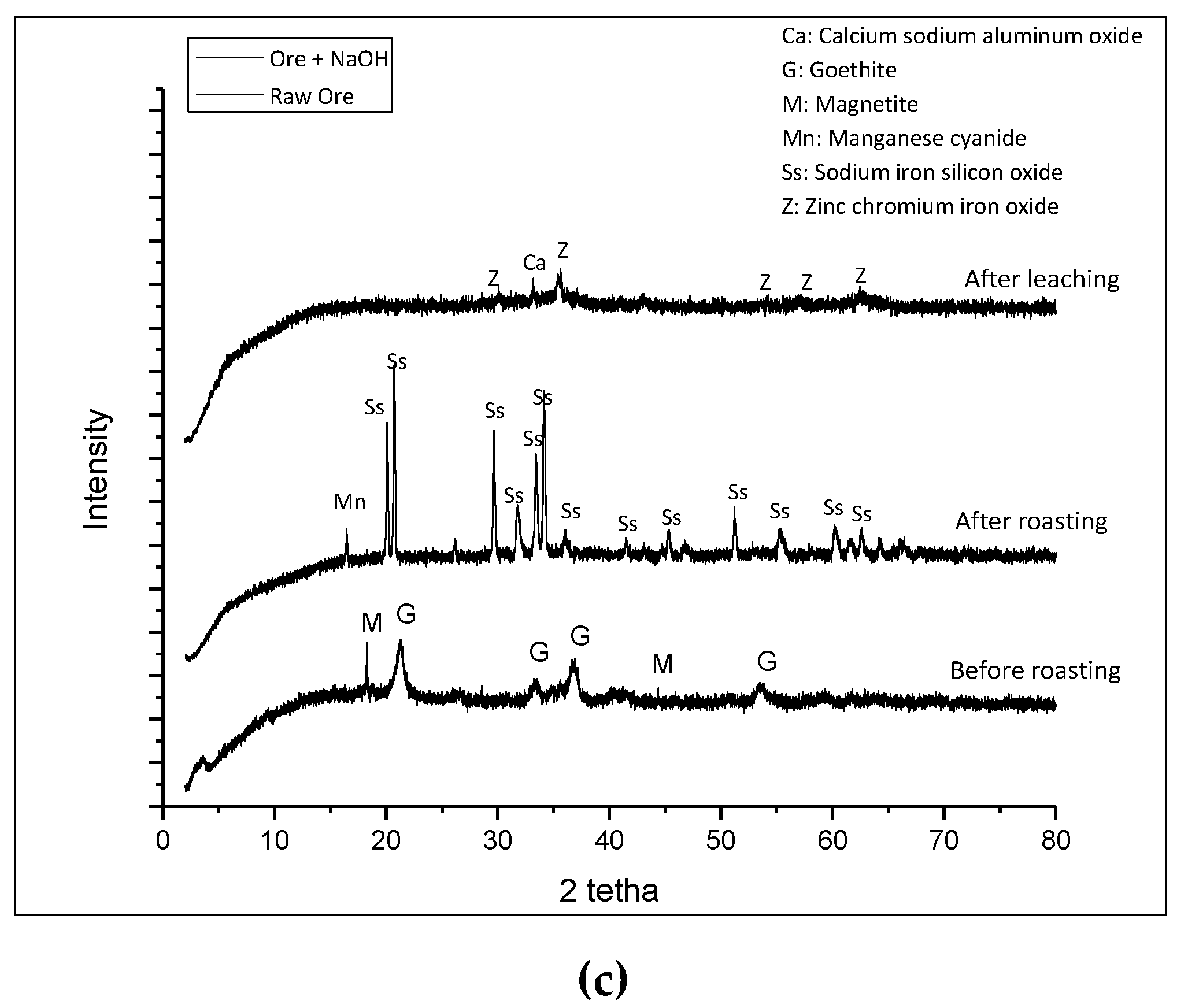
Minerals Free Full Text The Effect Of Alkali Roasting Pretreatment On Nickel Extraction From Limonite Ore By Using Dissolved So2 Air Html

Acids Bases And Salts Obuchenie Himii Himicheskie Eksperimenty Himiya
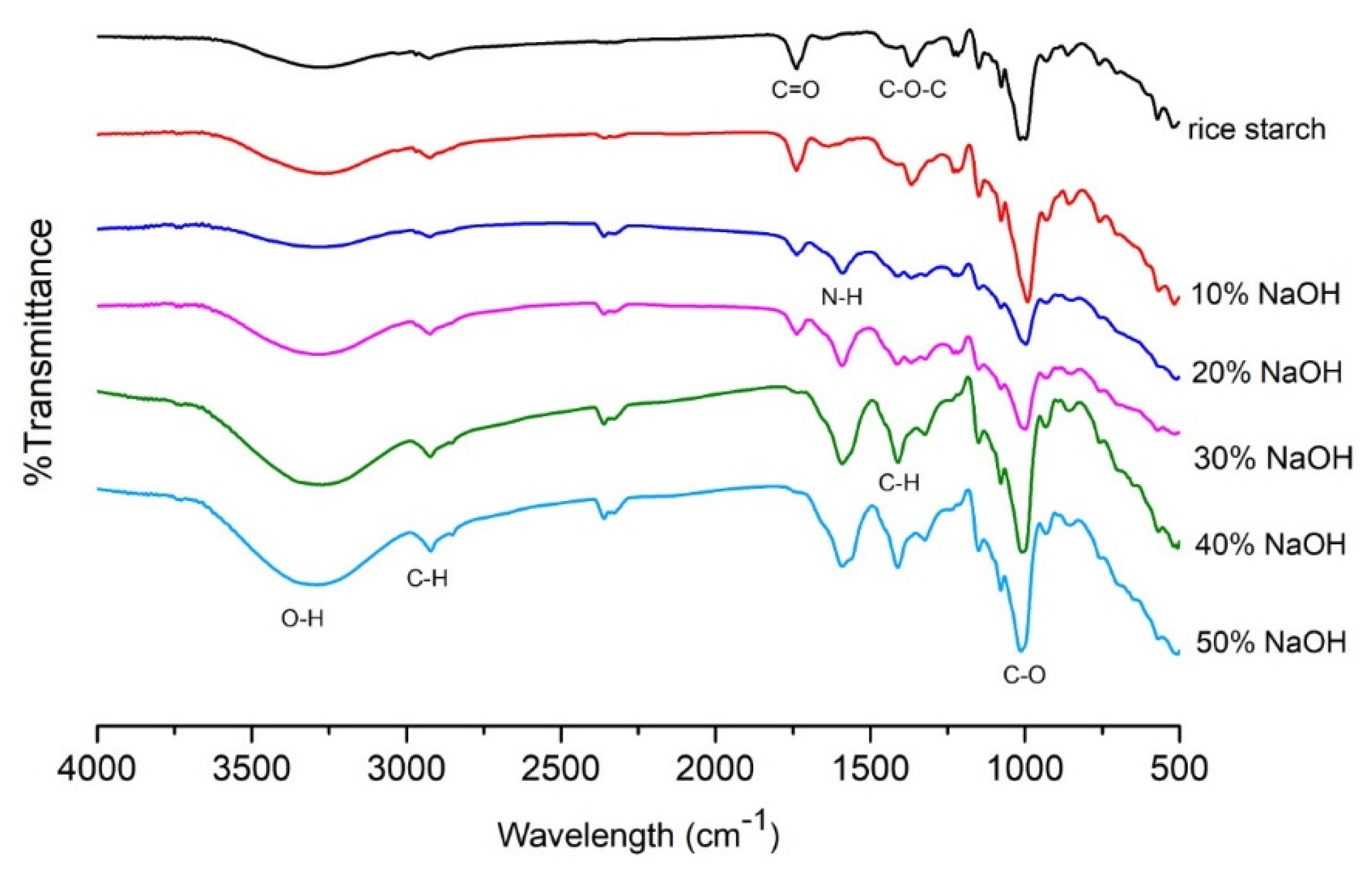
Molecules Free Full Text Morphology Mechanical And Water Barrier Properties Of Carboxymethyl Rice Starch Films Sodium Hydroxide Effect Html

The Ftir Spectra Of Freeze Dried A And Naoh Hcl Modified B Apple Pomace Download Scientific Diagram
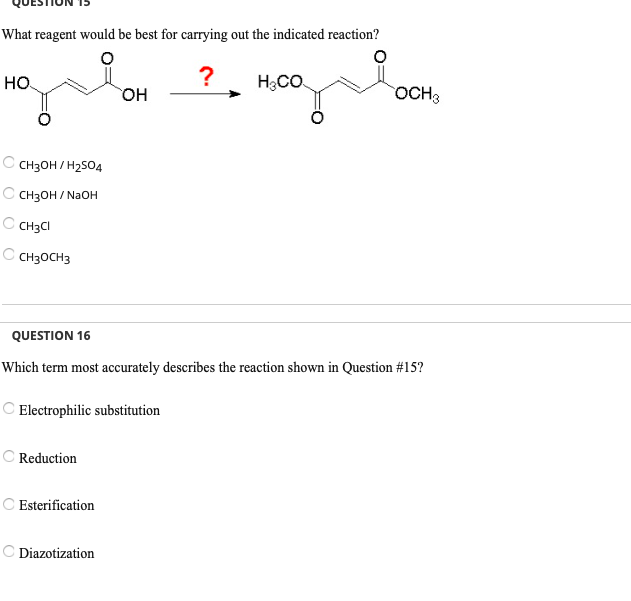
Solved Questiun 13 What Reagent Would Be Best For Carrying Chegg Com

Comments
Post a Comment